How Is Natural Gas Formed? From Geological Evolution to Energy Use
What is Natural Gas?: Definition and Classification
From a chemical perspective, natural gas is a hydrocarbon mixture primarily composed of methane (CH₄), accounting for more than 85% of its composition. This simplest organic molecule gives natural gas its clean-burning and high-efficiency characteristics. It also contains small amounts of ethane, propane, butane, and non-hydrocarbon gases such as nitrogen and carbon dioxide.
To better understand and utilize natural gas, it is typically classified by occurrence state and composition:
| Type | Occurrence / Composition | Extraction Difficulty & Technology | Examples / Usage |
|---|---|---|---|
| Conventional Natural Gas | Stored in porous rock formations (sandstone, limestone), similar to a “sponge” structure | Relatively easy to extract | Non-associated gas fields, associated gas with oil |
| Unconventional Natural Gas | Stored in tight rock formations, extremely small pores require special technology | Requires hydraulic fracturing, horizontal drilling | Shale gas, tight sandstone gas, coalbed methane |
| Dry Gas | Mainly methane with very little condensable liquids | Simple processing | Directly used as pipeline fuel |
| Wet Gas | Contains more liquid hydrocarbons (propane, butane) | Liquids must be separated first | Feedstock for LPG production |
| Sour Gas | Contains high levels of H₂S and CO₂ | Requires acid gas removal and corrosion prevention | Fuel or chemical feedstock after treatment |
| Sweet Gas | Low acid gas content | Low processing cost, directly usable | Stored in tight rock formations, extremely small pores require special technology |
What Conditions Are Necessary for the Formation of Natural Gas?
The formation of natural gas is not a random event; it requires a stable and long-term geological environment, like a precise “underground factory”. Its formation must go through three steps:
Step 1: Organic Matter Accumulation (Source Rock)
Dead plankton, algae, and plants are deposited in ancient lakes or oceans, mixing with mud to form organic-rich sediments—the foundation for future natural gas.
Over time, these layers become source rocks (also known as kerogen-rich shale), which are the raw materials for oil and natural gas.
Step 2: Burial and Transformation (Heat and Pressure)
As time passes, these sediments are buried deeper. Increasing temperature and pressure trigger chemical reactions that convert the organic matter into hydrocarbons.
Step 3: Migration and Trapping (Reservoir and Cap Rock)
The gas enters a porous reservoir (like sandstone) and is sealed under an impermeable cap rock (like shale or salt rock), preventing it from escaping.
Factors Influencing Natural Gas Formation
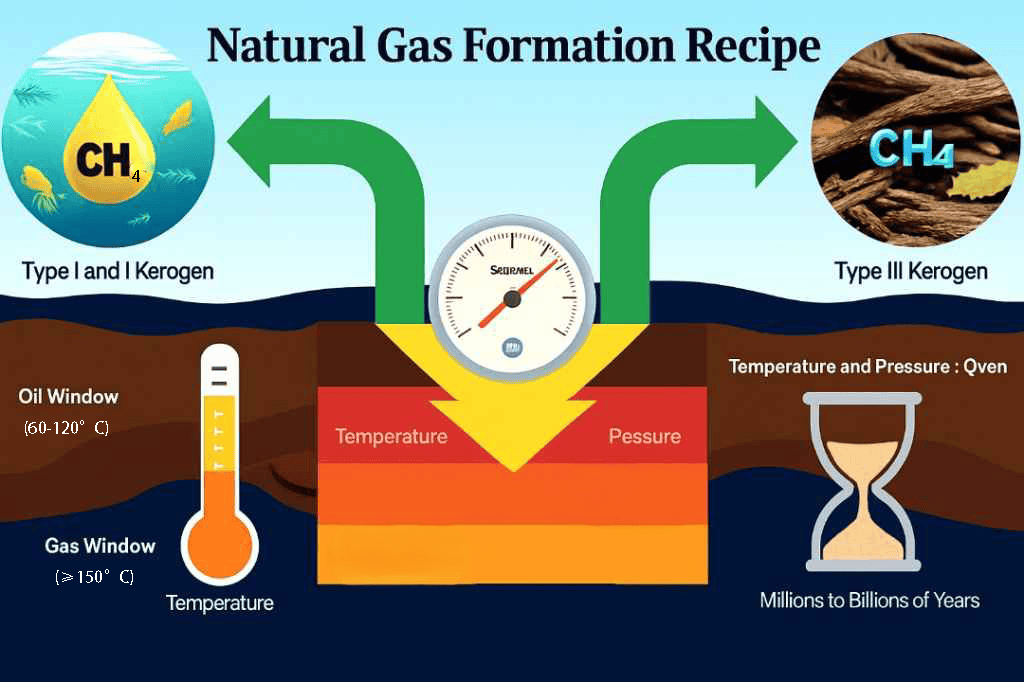
Several geological factors collectively determine whether natural gas can form, how much is produced, and its quality.
Temperature and Pressure: These are the “catalysts” and “pressure valves” for the conversion of organic matter into natural gas.
At relatively low temperatures (about 60-120°C), organic matter primarily generates oil, a stage known as the “oil window”.
When the temperature increases further (above 150°C), the existing oil or residual organic matter undergoes secondary cracking, producing large amounts of methane, entering the “gas window”. The higher the temperature, the higher the purity of the methane.
Type of Organic Matter: The origin of the organic matter determines whether the product is more likely to be oil or natural gas. Organic matter from aquatic plankton (Type I and Type II kerogen) can produce both oil and gas, while organic matter from terrestrial higher plants (Type III kerogen) tends to directly generate natural gas.
Time: This is an indispensable dimension. The conversion of organic matter is an extremely slow chemical process, typically requiring millions or even hundreds of millions of years to complete.
Tectonic Movement: Earth’s crustal movements (such as faults and folds) can create trapping structures for natural gas, but they can also destroy existing gas reservoirs, causing the gas to escape.
Detailed Explanation of Natural Gas Formation: A “Subterranean Kitchen” of Eons
Let’s connect these elements to take a detailed look at how natural gas is “cooked” in the “underground kitchen”:
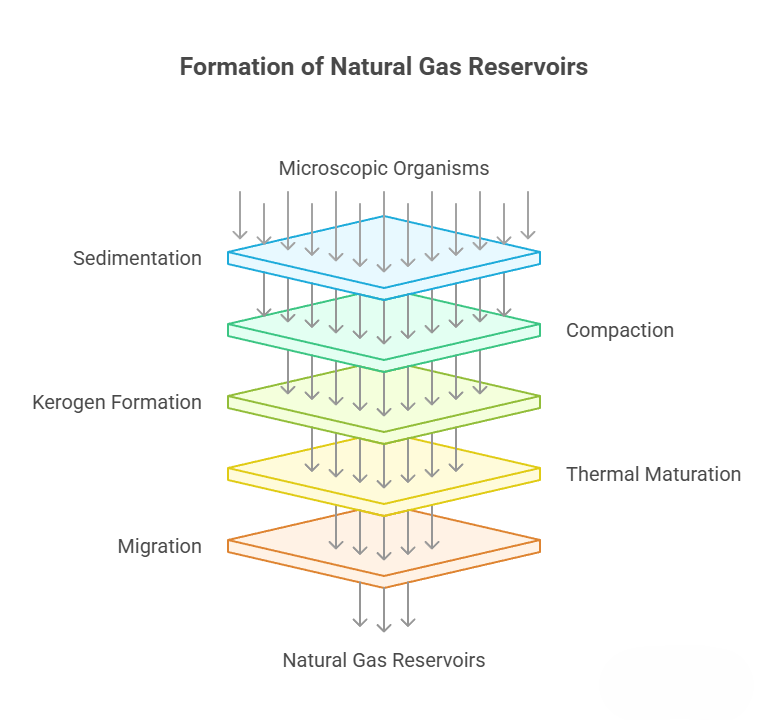
Sedimentation and Bacterial Decomposition Stage: Hundreds of millions of years ago, in vast lakes or oceans, countless tiny organisms died and sank to the bottom. In an oxygen-poor environment, their soft tissues were not completely decomposed and were deposited along with mud and sand.
Compaction and Kerogen Formation Stage: As the overlying sediments became thicker, this layer of organic-rich sedimentary rock was compacted, and the temperature and pressure began to rise. The organic matter, under the action of microbes and thermobaric effects, was converted into a complex solid organic substance called kerogen, the “precursor” to oil and natural gas.
Thermal Maturation and Cracking Stage (Oil and Gas Generation): As burial depth continued to increase, when the underground temperature reached above 60°C, the kerogen began to “cook”.
Oil Window (~60-120°C): Kerogen first cracks into long-chain liquid hydrocarbons, which is crude oil.
Wet Gas and Condensate Stage (~120-150°C): At higher temperatures, crude oil begins to crack, producing lighter hydrocarbons, forming wet gas and condensate.
Gas Window (>150°C): At even higher temperatures, both the residual kerogen and the early-formed oil undergo a dramatic chemical bond cleavage, ultimately cracking into the most stable, smallest-molecular-weight methane, which is dry gas.
Migration and Accumulation Stage: The generated gas is lighter than the surrounding rock and water. Driven by pressure differences and buoyancy, it “escapes” from the dense source rock and migrates upward or sideways. When it encounters a trap formed by a porous reservoir and a dense cap rock, it stops and accumulates, forming the natural gas reservoirs we can exploit today.
Where is Natural Gas Distributed?-Russia and Iran
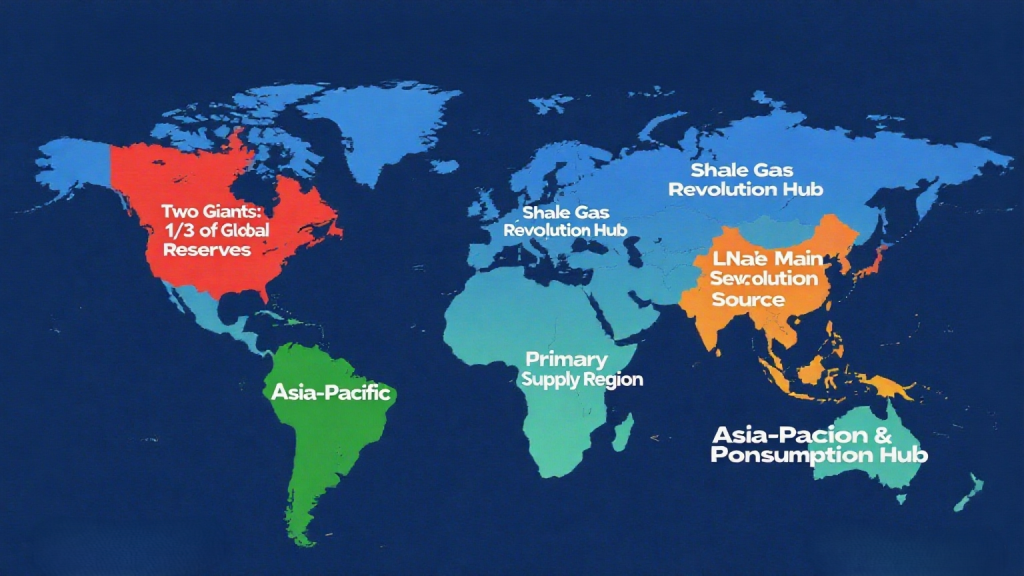
The distribution of natural gas is extremely uneven, concentrated in a few countries and regions with excellent resource endowments. According to the latest reserve data, the global natural gas landscape is as follows:
The Two Giants: Russia and Iran possess the world’s largest natural gas reserves, accounting for nearly one-third of the global total.
The Middle East: Besides Iran, countries like Qatar, Saudi Arabia, and the United Arab Emirates also have world-class natural gas reserves and are the main supply sources for liquefied natural gas (LNG) globally.
North America: Thanks to the shale gas revolution, the United States has the world’s highest natural gas production, and together with Canada, forms the North American natural gas market.
Asia-Pacific: China, Australia, and Turkmenistan are important natural gas producers and consumers in this region.
Natural Gas Extraction and Utilization: From Underground to Households
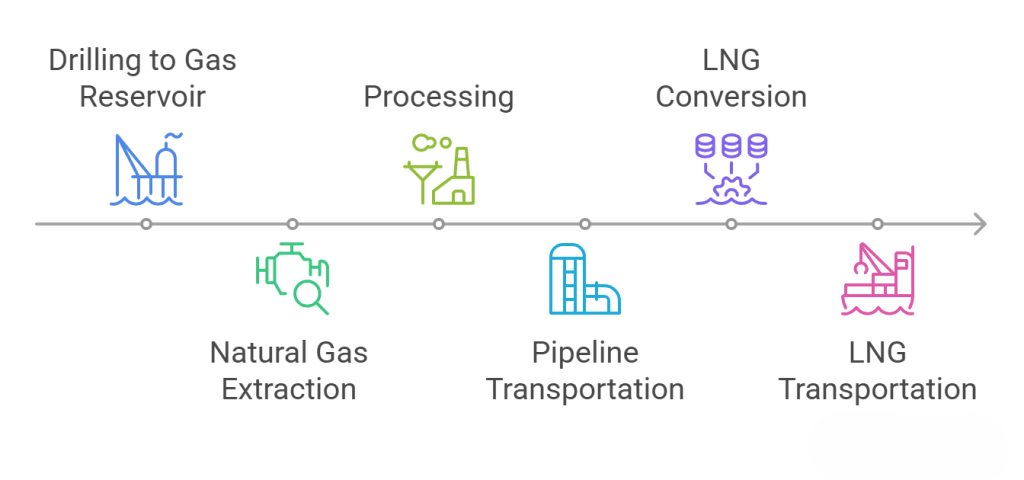
Extraction: After drilling into the gas reservoir, the natural gas is brought to the surface using the pressure of the formation itself. For unconventional resources like shale gas, hydraulic fracturing is used, which involves injecting high-pressure fluid underground to create artificial fractures in the rock to release the trapped natural gas.
Processing and Transportation: Extracted natural gas must first be processed to remove impurities like water, sulfides, and heavy hydrocarbons to meet usage standards. It is then transported in two main ways:
Pipeline Transport: Suitable for long-distance, large-scale onshore transportation.
Liquefied Natural Gas (LNG): Natural gas is cooled to -162°C, turning it into a liquid with a volume 1/600th of its original size, and then transported by specialized LNG carriers for long-distance shipping.
Utilization: Natural gas has a wide range of uses and is an essential energy source for modern society.
| Usage Category | Specific Applications | Features or Notes |
| City Gas | Residential cooking, water heating, and heating | Clean, efficient, and widely used in daily life |
| Power Generation | Gas-fired power plants | Flexible startup and shutdown, low emissions, a key means for peak-shaving and supporting renewable energy grid integration |
| Industrial Fuel | Glass, ceramics, metallurgy, and other industries | Provides a stable, high-calorific heat source, suitable for continuous industrial production |
| Chemical Feedstock | Production of ammonia, methanol, ethylene, etc. | An important basic raw material for organic chemistry, widely used in the chemical industry chain |
KAITIANGAS: Your Professional Partner for Distributed Natural Gas Recovery

Founded in 2002, KAITIANGAS specializes in the recovery and treatment of distributed natural gas resources. The company focuses on the development, engineering design, and global promotion of distributed natural gas recovery and liquefaction technologies and equipment. KAITIANGAS holds independent intellectual property rights, mature system solutions, and a professional team, and has successfully built and operated multiple wellhead LNG recovery projects in China, the Middle East, and Southeast Asia, accumulating extensive on-site experience and technical advantages.
Environmental Impact and Low-Carbon Value of Natural Gas
In the context of global climate change, the environmental attributes of natural gas are receiving significant attention.
Environmental Challenges: Although it is clean, methane, the main component of natural gas, is a potent greenhouse gas itself. Therefore, controlling methane leaks throughout the entire chain of extraction, transportation, and use is crucial and a prerequisite for realizing its low-carbon value.
Low-Carbon Advantage: Compared to coal and oil, natural gas is the cleanest fossil fuel. Its main component, methane, has a low carbon and high hydrogen content. Its combustion produces about 50% less carbon dioxide than coal and about 30% less than oil, with almost no sulfur oxides or particulate matter.
“Bridge Fuel” Role: In the transition from a high-carbon fossil fuel era to a zero-carbon era dominated by renewable energy sources like wind and solar, the energy system needs stable and flexible power sources for transition and support. Gas-fired power generation can play this “bridge” role, compensating for the intermittency and instability of wind and solar power and ensuring grid security.
Conclusion and Outlook
From microscopic life billions of years ago, to the organic treasure buried deep underground, to the clean flame that lights up modern life, the formation of natural gas is a magnificent epic of Earth’s evolution. It is not only a masterpiece of nature’s craftsmanship but also an important driving force for the development of human society.